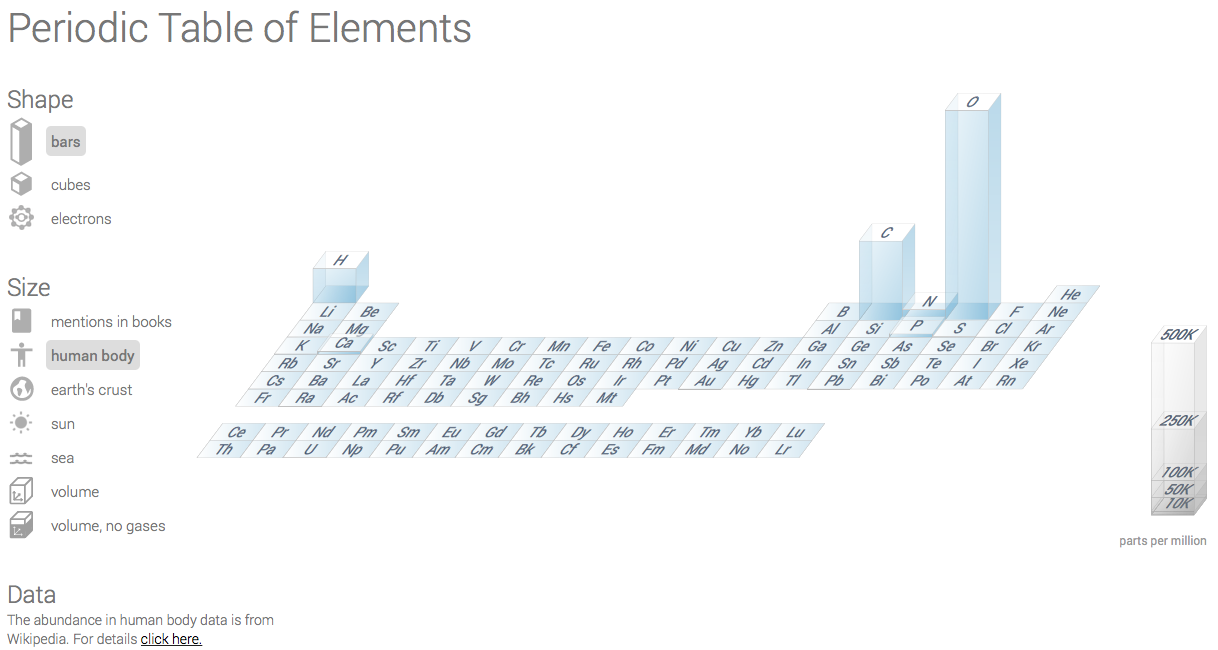
Today Google Research posted an interactive periodic table of elements with statistics about the abundance of different elements in, among others, the earth’s crust, the sea and in literature. One of them, showing the the abundance of elements in humans (Figure 1), looks a bit weird… it says 61% of all atoms in the human body are oxygen, 23% carbon, 10% hydrogen and 3% nitrogen. This can not be correct.
Figure 1: Periodic table of elements with the abundance of atoms in the human body. It is WRONG!
A quick back-of-the-envelope calculation:
- half of the human body weight is water (2/3 hydrogen and 1/3 oxygen)
- the other half is proteins (amino acids), lipids (fat), DNA and other stuff. Most of them have a carbon backbone. Intuitively I would say that most carbons have one or two hydrogens attached and only a few have oxygen attached.
In both cases there is much more non-oxygen than oxygen… so where does the oxygen come from?
The Google Research page refers to a Wikipedia page about the composition of the human body [1]. A quick look at the table makes the problem clear: Google used mass percentage instead of atomic percentage (or mole percentage). Every school kid knows that this makes quite a difference. For example, water is H2O. The atomic percentage oxygen is 33%. Because oxygen is 16 times heavier than hydrogen the mass percentage of oxygen in water is 89%.
It is of course nice to prove Google wrong. It is also nice that Wikipedia gives you an answer, but I was wondering how accurate my back-of-the-envelope estimation would have been.
Let’s take a person who weighs 100 kg and contains 53 mass-% water. With a molar mass of 18 g/mole this means there is ~2950 mole of water. This means 2950 mole of oxygen and 5900 mole of hydrogen. [2]
The other 47 mass-% of the human body is more difficult. Totally unscientifically I decided to only consider proteins (actually: amino acids). Even more unscientifically I will only consider the protein backbone, with two carbons, an oxygen, a nitrogen and three hydrogens. This adds up to a molecular weight of 57 g/mole, giving 824 mole of “other stuff”. Using these numbers we can calculate the moles, mass and mole-%. Table 1 summarizes the results and compares them with the data found on Wikipedia.
| Element | Molar mass | Mole | Mass (kg) | Mole-% | Mole-% |
|---|---|---|---|---|---|
| (g/mole) | (Estimation) | (Wikipedia) | |||
| C | 12 | 1649 | 19.8 | 11.3 | 12 |
| O | 16 | 3769 | 60.3 | 25.8 | 24 |
| N | 14 | 824 | 11.5 | 5.6 | 1.1 |
| H | 1 | 8362 | 8.4 | 57.3 | 62 |
| Total: | 14605 | 100 | 100 | 99.1 | |
Table 1: Abundance of elements, for 100 kg person with 53 mass-% water.
Frankly, the results are freakishly close. The estimation adds up to 100 mole-% because I didn’t consider the rest of the periodic table (which is only 0.9 mole-%). The calculation overestimates the mole-% nitrogen and underestimates the mol-% hydrogen because I neglected the lipids. Lipids (fat) are long chains of carbons with one or two hydrogens attached and hardly any nitrogens.
How many lipids does a person have? Like water, this depends on the person. Wikipedia also has a list with the composition by molecule type. It is difficult to compare the results with the data above because this table assumes 65 mass-% water (Table 2 shows the results of my estimation with 65 mass-% water). In any case, the Wikipedia table says humans have 20 mass-% proteins and 12 mass-% lipids.
| Element | Molar mass | Mole | Mass (kg) | Mole-% |
|---|---|---|---|---|
| (g/mole) | (Estimation) | |||
| C | 12 | 1228 | 14.7 | 8.1 |
| O | 16 | 4225 | 67.6 | 27.9 |
| N | 14 | 614 | 8.6 | 4.1 |
| H | 1 | 9064 | 9.1 | 59.9 |
| Total: | 15131 | 100 | 100 | |
Table 2: Abundance of elements, for 100 kg person with 65 mass-% water.
What did I learn from this?
- Google is not infallible.
- My back-of-the-envelope calculation is surprisingly accurate.
- The mass-% of water in humans obviously differs. Wikipedia is not very consistent in using a particular percentage or indicating what percentage was used.
- 4 elements make up more than 99 mole-% (96 mass-%) of the human body. That is kind of amazing.
- The next most abundant elements are calcium and phosphorus, with 0.22 mole-% each (1.4 and 1.1 mass-% respectively). I had expected that humans would contain much more calcium. If not calcium, what are bones made of?
[1] Google Research uses Wikipedia as a source? Seriously? ↩
[2] A mole is shorthand for a large number. If you have 1 mole of bikes and you take them apart, you have 1 mole of steers and 2 moles of wheels. ↩